A common challenge in many T cell therapy strategies is the quality of the T cells being produced. In principle, even a few 100 T cells of the right specificity AND quality should be capable of expanding to many millions of potent killer T cells upon activation, eradicating their targets and forming memory. Unfortunately, for many cell therapy approaches, the industry relies on ex vivo expansion of these cells. For example, a typical TCR, CAR, or TIL therapy is produced by expanding T cells outside of the body for many days. Due to the challenges of recreating the intricate signaling events that happen in a healthy human - the products of T cell therapy manufacturing (especially for most TCRs and TILs) are exhausted. This inferior T cell quality often necessitates steps such as pre-conditioning, cytokine administration and high dosing levels to partially compensate for this lack of T cell quality with pure quantity.



A major question is therefore, how do you train your T cells to be good at killing (cytotoxicity), more proliferative and more likely to form memory. While the field has dramatically improved its knowledge of underlying T cell signaling cascades that drive certain phenotypes, manipulating them remains a challenge. We believe that one can convert exhausted T cells into far more productive naive or memory-like cells simply by delivering the right transcription factors.
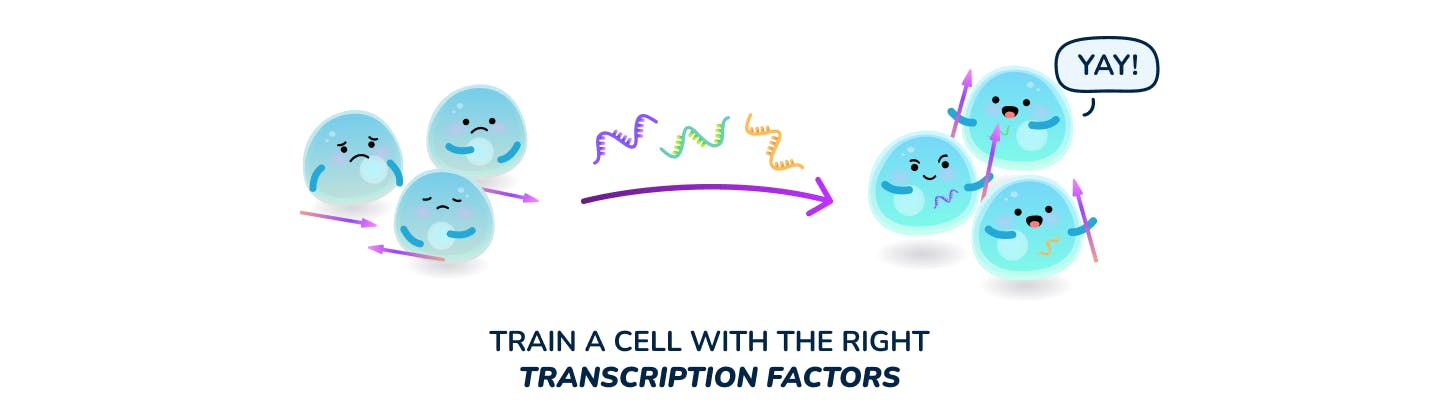

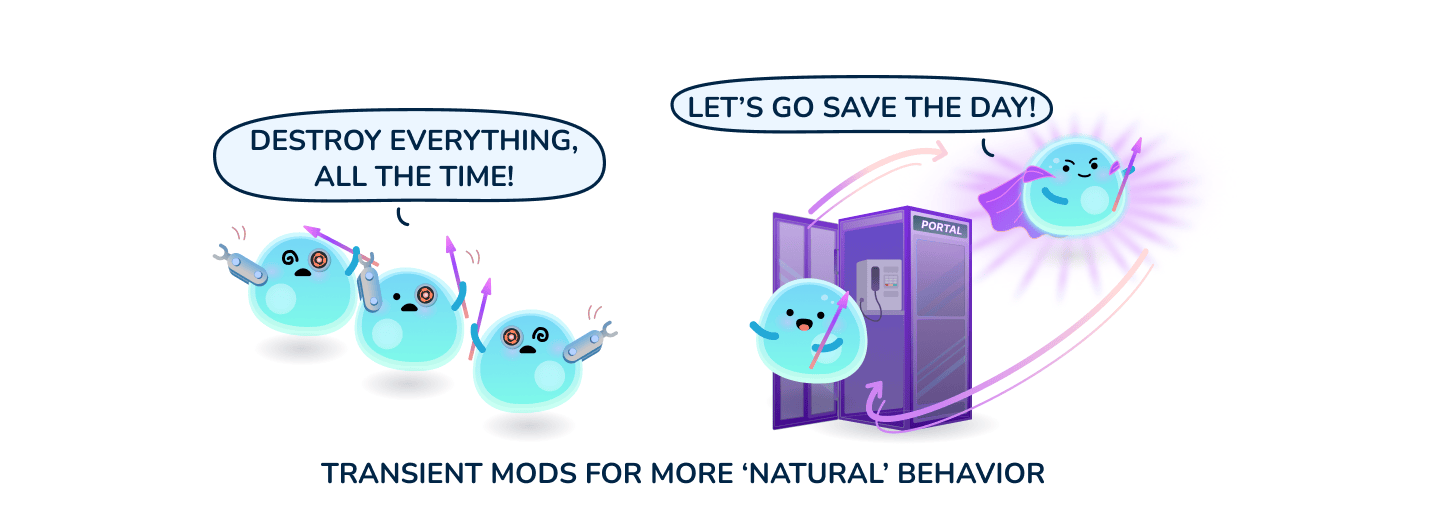
To train a cell, one can deliver transcription factors in mRNA form and also use tools like siRNA to knockdown undesirable traits. By leveraging the right mix of factors, one can likely convert the T cell to its desired phenotype and kickstart the cell signaling feedback loops that make this state stable. While gene knockout or knock-in could also be used to alter some T cell behavior, it can be a double-edged sword. Due to the permanent nature of genetic modifications, cells may be unnaturally locked into certain behaviors that interfere with the normal cascade of expansion and contraction of an immune response - thereby risking significant hyper immune side-effects. Some historical clinical trials leveraging genetic modification of T cell enhancing domains resulted in fatal toxicities.
By delivering the right combination of non-genetically modifying cargos, particularly mRNA and siRNA, one could potentially convert exhausted T cells into much more productive memory cells. A successful strategy has the potential to dramatically improve efficacy, reduce or eliminate the need for concurrent conditioning regimens, and provide long-term surveillance/protection against tumor recurrence.